Can Heart Simulations Enable Better Clinical Outcomes?
By Chuck Seegert, Ph.D.
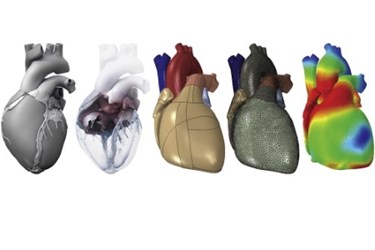
Imagine a virtual heart that would enable surgical planning and simulation testing of new medical devices before they are clinically tested. This is the ambitious goal of a new project from Dassault Systèmes, and the French company has enlisted the support of industry, clinicians, and even the FDA.
Virtual modeling is a technique widely used in the aerospace and automotive industries to help predict the performance of new designs. In silico testing like this is undertaken before investing in costly development projects that may not work. Confidence in these types of models comes from a method called validation where, through extensive testing that often includes in-depth statistical analysis, the model is shown to reliably perform in a way that replicates reality. If this is successful, then tests run in the model give a good prediction of what will happen when they are performed physically and can reduce the actual testing required in the lab.
Dassault is the publisher of computer-aided design (CAD) packages like SolidWorks, and is well known for its 3D modeling expertise. Recently, with the announcement of the Living Heart Project (LHP), the company decided to use its core competency to tackle a challenge that has proven insurmountable to date: modeling the living heart.
“Thirty percent of all people die from heart disease,” said Steve Levine, chief strategy officer at Dassault and director of the Living Heart Project, in a recent article from the New York Times (NYT). “We’re not winning the war. It’s getting worse. There’s a lot of cool stuff happening, but it’s not translating into better health and longer life.”
A key requirement of the system is that it accurately reflects the reality of the heart, which can be used to test pacemaker leads and other medical devices. To achieve this, Dassault has entered into a 5-year research agreement with the FDA. The agency will provide regulatory input for the project, helping to guide it to a conclusion that will be useful in the eyes of the agency, according to a recent press release from Dassault.
This agreement with the FDA is just the latest step in the project, which has engaged with 30 contributing organizations and over 100 cardiovascular specialists to develop its model system, according to the press release. This kind of involvement from industry, clinicians, and regulatory bodies may be unprecedented in the medical device space, which is an indication of the commitment Dassault is bringing to the project.
“Computational modeling and simulation has the potential to revolutionize the medical device and healthcare fields by accelerating innovation and providing comprehensive evidence of long-term safety,” said Bill Murray, president and CEO of the Medical Device Innovation Consortium, in the Dassault press release.
While Dassault continues to drive forward the area of predictive testing for medical devices, improving predictive diagnostics for heart failure through analytical methods is another important area of research.
Image Credit: “The Living Heart Project: A robust and integrative simulator for human heart function,” European Journal of Mechanics – A/Solids, CC By-NC-ND 3.0: http://creativecommons.org/licenses/by-nc-nd/3.0/
