Voices of Biotech
Podcast: MilliporeSigma says education vital to creating unbreakable chain for sustainability
MilliporeSigma discusses the importance of people, education, and the benefits of embracing discomfort to bolster sustainability efforts.
Sponsored by Sinfonia

CellQualia ICP System
HTTPS://WWW.CELLQUALIA.COM/ENG
Cells are used as raw materials, intermediates, and final products in biopharmaceutical and cell therapy manufacturing. Because living cells are always in a dynamic state, their characteristics must be kept within specified ranges throughout bioprocessing to preserve their utility. Cell population expansion without changing the original cell properties is key for obtaining the required number of cells at the next step. However, limited process data are collected during the expansion phase — information that could be used to understand and control fully this important step.
Two major cell types used in cell therapy manufacturing are mesenchymal stem cells (MSCs) and pluripotent stem cells (PSCs) such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). MSCs are obtained from donors and expanded without processing before administration to patients. PSCs are expanded to make master and working cell banks (MCBs and WCBs), which are used as starting materials for directed differentiation to obtain desired cell types.
Cell expansion might appear to be a simple procedure in which cells are grown in culture media in a controlled environment. Thus, a quality check (QC) generally would need to be applied only at the final step in the form of quality by testing (QbT). In reality, however, cell expansion is a multiparametric and complex process. Deviations can come from different sources, including raw materials (e.g., cell donor, tissue origin, cell type, culture media, supplements, and culture vessels), processing methods (isolation, expansion, and harvest), environment (temperature, oxygen, and pH), and human interventions (working time and day, operator skills and errors).
The CellQualia intelligent cell processing (ICP) System (photo above) is a new product manufactured by Sinfonia Technology Co., Ltd. It was developed in collaboration with Shin Kawamata at the Foundation for Biomedical Research and Innovation at Kobe (FBRI) and is an automated instrument for cell expansion with process analytical technologies (PATs). Below, we discuss how the system is designed to be a platform device for helping biomanufacturers realize quality by design (QbD).
The Need for Automation
Most, if not all, cell manufacturing still is implemented and recorded manually following standard operating procedures (SOPs). Skilled operators play an important role in a company’s efforts to reduce deviations in cell manufacturing. The simplest indications of cell-culture health are culture-media color, cellular morphology, and cell growth rate. Traditionally, assessing those parameters has been sufficient for skilled staff to determine the timing of media exchange, subculturing, and harvesting. The frequency of errors that come from such activities can be decreased with repeated operator training and self-checking. But skill and experience are unique to each individual, and it takes time to train new staff. For those reasons, automated and intelligent systems are needed as alternatives to manual manufacturing by skilled staff. Automation also will improve financial considerations by reducing labor costs.
Instrumentation Over Robotization
Automation can be realized through robotization and instrumentation. A robot is suitable for full mimicking of manual operations such as material handling and liquid pipetting, without significant changes to manufacturing procedures. An instrument consists of mobile units that transform each operation into simple movements, so manufacturing steps need to be modified to fit with the instrument used. Aseptic operations and sterilization steps are required for both manual and robotic manufacturing because they are open operations. However, an instrument does not require such sterilization steps if it is designed as a closed system.
Culture-Status Monitoring
Environmental data, temperature, humidity, oxygen, and carbon dioxide are monitored and recorded during cellular incubation. Occasional microscopic observation is informative but is not a quantitative assessment of culture status. Skilled staff must make decisions regarding cellular morphology and confluency, and artificial intelligence (AI) can help them deliver a more objective assessment. For example, a camera can be placed at the bottom of culture vessels at incubation, so that both robots and instruments can be equipped with imaging systems. Glucose consumption, lactate accumulation, and pH of culture media are parameters for assessing cellular vitality and metabolic status, and they can be measured with chemical sensors in a liquid phase. Because the installation of sensors in typical culture vessels is impractical for reasons of flexibility, maneuverability, and cost, application of in-line sensors in an instrument’s liquid transfer line is preferred. Media and cell analyses by occasional sampling also are informative if they are available.
Early Signs of Cellular Quality Change
Stem cells in the human body are quiescent and rely on glycolysis to prevent them from oxidative stress. However, in vitro expansion of stem cells prompts them to enter the cell cycle, increase mitochondrial biogenesis, and shift from glycolysis to oxidative phosphorylation (OXPHOS). MSCs expanded under aerobic conditions generate a significant proportion of adenosine triphosphate (ATP) through OXPHOS (1), but they are still highly glycolytic (2). Human PSCs also rely mostly on glycolysis to meet their energy demands (3) and will die by glucose depletion (4). Lactate is an end product of glycolysis that is known to have negative impacts on cultured cells at high concentrations. The gene expression profile of human MSCs can be influenced by lactate in a dose-dependent manner (5). Accumulation of lactate in culture media has been shown to decrease the growth rate of PSCs regardless of pH control (6) and correlates with incidence of both DNA damage and genomic alterations (7). As described above, the metabolic state of stem cells is an important point to be noted in their culturing and an early indication of their quality change. In general, however, metabolic state is not monitoried as a parameter either in manufacturing or in cell maintenance and banking.
In human PSCs, spontaneous differentiation can cause degeneration, and cellular metabolic change is identified as an early indicator of PSC differentiation. Kynurenine in a culture medium is a biomarker for the undifferentiated state, and 2-aminoadipic acid (2-AAA) is a biomarker indicating commitment to differentiate along the ectoderm lineage (8). A routine method of their measurement is liquid chromatography with mass spectrometry (LC–MS), which is noninvasive, so those biomarkers are suitable for process monitoring.
Application of CellQualia ICP System to Cell Manufacturing
Because a change in cellular quality is caused or indexed by the cellular metabolites, biomanufacturers should monitor those throughout cell manufacturing. The CellQualia ICP system is equipped with both in-line sensors and an autosampling function to measure the temporal change of cellular metabolite concentration and of a culture environment.
In our pilot study, consumption of glucose, accumulation of lactate, and concomitant change of pH in culture media were monitored (Figure 1). Lactate was identified to be useful as a surrogate for cell counting in determining the autopassaging timing of both MSCs and iPSCs. During culturing of human iPSCs, kynurenine accumulated over time, and the concentration of 2-AAA remained at a low level (Figure 1), thus suggesting that the cells retained their undifferentiated status throughout automated culturing and passaging. Standard quality control testing of human MSCs (9) and iPSCs (10) was performed to validate cell quality at autopassaging and harvesting and for thawed cells after cryopreservation (Figure 1, Table 1).
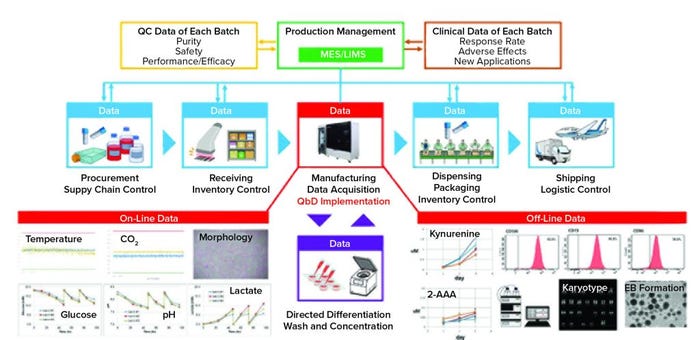
Figure 1: An integrated cell manufacturing platform includes CellQualia intelligent cell processing (ICP) system. Each part of the supply chain generates data, making the control of final-quality cellular products a complex task. Manufacturing is the most important and complicated element. Data for on- and off-line analyses are shown as examples. MES is manufacturing execution system, LIMS is laboratory information management system, 2-AAA is 2-aminoadipic acid. EB is embyoid body.
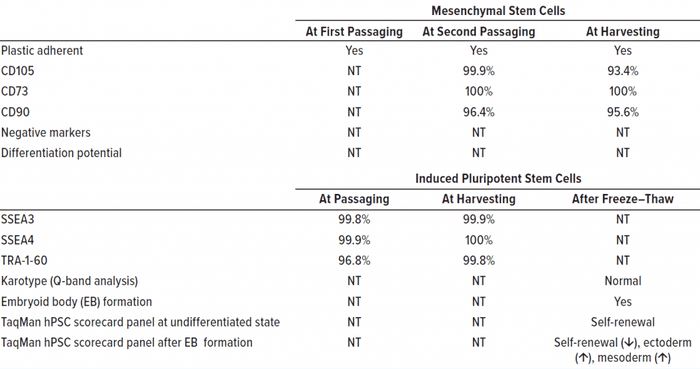
Table 1:Results of standard quality control testing are listed for human mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) expanded with a CellQualia intelligent cell processing (ICP) system, with representative batch data as an example. MSCs were expanded serially with CellSTACK (from Thermo Fisher Scientific) one chamber and five chamber, and HYPERStack (from Corning) 36-layer flasks. iPSCs were expanded using CellSTACK two- and
10-chambers. “NT” is not tested.
No microbial contamination has been reported with the use of the CellQualia ICP system so far, even when it was placed and operated in a standard biochemical laboratory. Installing the system in a downgraded environments can reduce a company��’s construction and maintenance costs significantly.
The CellQualia ICP system also enables end users to accumulate data from human MSCs and iPSC expansion cultures as conditions change, including seeding densities, donor or batch of seed cells, and type or lot of culture media and reagents. The resulting data help developers assess critical attributes for risk-based manufacturing control, enabling quality by design (QbD). In general, cell washing, concentration, directed differentiation, and dispensing will follow cell expansion with a CellQualia ICP system as needed and generate data as well. A detailed discussion of those elements is for another study.
Scale-Up or Parallel Manufacturing
Scale-up is an important process in the industrialization of cell manufacturing. To date, bioreactors can be used with microbeads or cells acclimated to suspension culture and hollow-fiber systems for cell culture scale-up, and the number or size of culture vessels can be increased. Because a change in cell culturing format can cause a significant change in a cellular environment and culture quality, inconsistency in manufacturing methods requires the confirmation of product equivalency and efficacy. On the other hand, a simple increment in the number or size of culture vessels will increase the amount of time, space, and staff headcount required. An extension of handling time also can compromise cell quality. Parallel manufacturing with multiple CellQualia ICP systems is a feasible solution. They can be used to scale up cell manufacturing for a single facility and at multiple sites, including at the bedside, with uniform product quality.
Realization of an Intelligent Cell Manufacturing System
As Figure 1 shows, manufacturing is part of the supply chain for cellular products. Product deviation can come from different sources. For example, preservation conditions and duration are critical for securing the high quality of both raw materials and cellular products. Inventory and logistic control of these are important to prevent errors in delivery of a manufactured therapy. Thus, the cell manufacturing industry must pay attention to the whole supply chain and control it to secure the quality of final products. It also is important to apply QbD to cellular products. Feedback on product quality also should be obtained from QC data of each batch and from clinical applications. Integration of all data from the supply chain for analysis and product improvement will make for a truly intelligent cell manufacturing process, and we believe the CellQualia ICP system can be the key component of that.
References
1 Pattappa G, et al. The Metabolism of Human Mesenchymal Stem Cells During Proliferation and Differentiation. J. Cell. Physiol. 226(10) 2011: 2562–2570; https://doi.org/10.1002/jcp.22605.
2 Nuschke A, et al. Mesenchymal Stem Cells/Multipotent Stromal Cells (MSCs) Are Glycolytic and Thus Glucose Is a Limiting Factor of In Vitro Models of MSC Starvation. Stem Cell. Res. Ther. 7(1) 2016: 179; https://doi.org/10.1186/s13287-016-0436-7.
3 Varum S, et al. Energy Metabolism in Human Pluripotent Stem Cells and Their Differentiated Counterparts. PLoS One 6(6) 2011: e20914; https://doi.org/10.1371/journal.pone.0020914.
4 Hemmi N, et al. A Massive Suspension Culture System with Metabolic Purification for Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells Transl. Med. 3(12) 2014: 1473–1483; https://doi.org/10.5966/sctm.2014-0072.
5 Schneider C-C, et al. Lactate Influences the Gene Expression Profile of Human Mesenchymal Stem Cells (hMSC) in a Dose Dependant Manner. Cell Physiol. Biochem. 30(6) 2012: 1547–1556; https://doi.org/10.1159/000343342.
6 Horiguchi I, et al. Effects of Glucose, Lactate, and Basic FGF as Limiting Factors on the Expansion of Human Induced Pluripotent Stem Cells. J. Biosci. Bioeng. 125(1) 2018: 111–115; https://doi.org/10.1016/j.jbiosc.2017.08.004.
7 Jacobs K, et al. Higher-Density Culture in Human Embryonic Stem Cells Results in DNA Damage and Genome Instability. Stem Cell Reports 6(3) 2016: 330–341; https://doi.org/10.1016/j.stemcr.2016.01.015.
8 Yamamoto T, et al. Kynurenine Signaling Through the Aryl Hydrocarbon Receptor Maintains the Undifferentiated State of Human Embryonic Stem Cells. Sci. Signal 12(587) 2019: eaaw3306; https://doi.org/10.1126/scisignal.aaw3306.
9 Dominici M, et al. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytother. 8(4) 2006: 315–317; https://doi.org/10.1080/14653240600855905.
10 Sullivan S, et al. Quality Control Guidelines for Clinical-Grade Human Induced Pluripotent Stem Cell Lines. Regen. Med. 13(7) 2018: 859–866; https://doi.org/10.2217/rme-2018-0095.
Corresponding author Masaki Hosoya, PhD, ([email protected]) is senior staff manager of business planning section, Tsutomu Soma, PhD, is manager and senior engineer, and Hirotoshi Kawamura is general manager and manager of business planning section, at the medical engineering center at Sinfonia Technology Co., Ltd.
You May Also Like